Laboratory Medicine | About Us
About Us
In response to disease diagnosis, therapeutic monitoring, and disease prevention, clinical laboratory testing has become an indispensable component of modern medical practice. By applying laboratory science methodologies, clinical testing provides objective and quantitative data or reports that reflect patients’ physiological conditions, thereby offering crucial medical evidence for confirmation, exclusion, guidance, and monitoring in patient care.
With continuous advances in medical technology, increasing patient demands, and progressively higher expectations for quality, rapid developments in testing technologies have greatly expanded the scope of clinical laboratory applications. In addition to traditional testing areas and services—such as routine examinations of liver function, renal function, blood, and urine—molecular diagnostics and precision medicine–related tests have gained increasing importance. These include genetic testing for cancer susceptibility, cancer risk genes, cerebrovascular and cardiovascular disease risk genes, as well as nucleic acid testing for SARS-CoV-2. By integrating emerging fields such as bioinformatics, these advanced technologies are gradually being applied and incorporated into clinical laboratory testing, enabling the provision of more personalized clinical laboratory services.
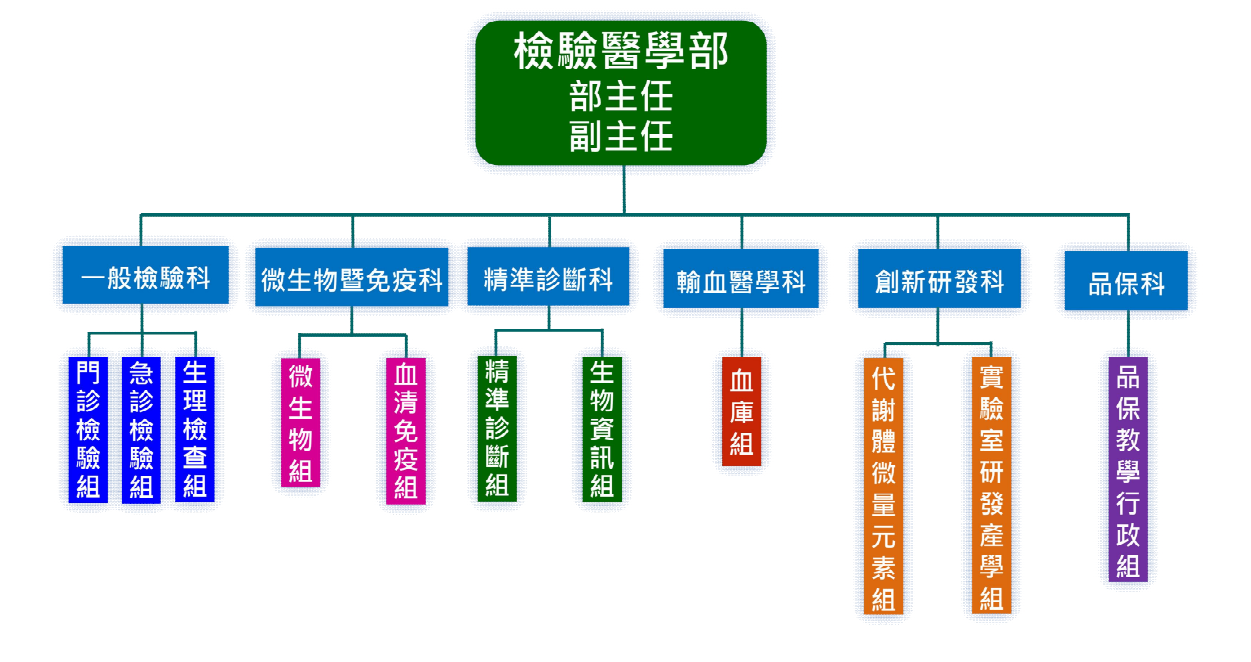
The Department of Laboratory Medicine currently comprises six divisions: General Laboratory, Microbiology and Immunology, Precision Diagnostics, Transfusion Medicine, Innovation and Research & Development, and Quality Assurance. Each division is led by a specialist physician or a senior medical laboratory scientist serving as division director. Together, they are responsible for planning, promoting, and managing the development of laboratory services, education, and research.
Under these divisions, the department operates elevent functional sections, including Section of Outpatient Service Laboratory, Section of Emergency Service Laboratory, Section of Physiological Examination (EKG), Section of Microbiology, Section of Serology and Immunology, Section of Precision Diagnosis, Section of Bioinformatics, Section of Blood Bank, Section of Metabolomics and Trace Elements, Section of Industry-academia Research and Development, and Section of Quality Assurance, Education and Administration. These sections are coordinated by technical supervisors who oversee the execution of laboratory operations and the control of testing quality and technologies.
The department primarily serves patients and physicians of this hospital, supports specialized laboratory testing for affiliated hospitals within the healthcare system, and assists hospitals and clinics of all levels in the central region through referral and outsourced testing services. In addition, the department implements observation and internship training programs for students from relevant university departments, and accepts applications for visits and advanced training from domestic medical institutions as well as international academic and healthcare professionals.
Other important services of the department include supporting or leading various international clinical trials, as well as conducting industry–academia collaboration projects and clinical research in partnership with biotechnology companies. From round-the-clock blood bank and emergency laboratory operations to routine health examination tests; from transplantation-related testing to personalized genetic testing; and from product validation and evaluation for biotechnology companies, the scope of services is extensive. The core values guiding overall operations and management are professionalism, accuracy, timeliness, and safety. All staff members work collaboratively to provide comprehensive and high-quality laboratory services, cultivate outstanding laboratory professionals, and uphold the mission of ensuring laboratory and patient safety.
In 2011, the department collaborated with the Children’s Medical Center and National Chiao Tung University to apply MALDI-TOF (Matrix-Assisted Laser Desorption Ionization Time-of-Flight) technology for the identification of variant hemoglobin amino acid sequences. In the same year, the department published research in an international scientific journal demonstrating the effectiveness of High-Resolution Melting (HRM) analysis for the differential diagnosis of β-thalassemia genotypes. This innovative integration of molecular diagnostic principles and advanced technologies was recognized in 2011 with the National Quality Award (Symbol of National Quality) by the Institute for Biotechnology and Medicine Industry, under the title “The Guardian Team for Thalassemia and Hemoglobin Gene Disorders.”
A Clinical Laboratory Meeting World-Class Standards
Our department is committed to maintaining a laboratory that meets ISO 15189 and CAP international standards, while continuously enhancing staff education, training, and team collaboration. We uphold accuracy, efficiency, professionalism, and safety in all aspects of our work.
Through our Division of Innovation, Research and Development , we develop and clinically validate advanced testing in precision molecular diagnostics, metabolomics, and trace elements, expanding diagnostic and therapeutic options for patients. We also provide a platform for industry–academia collaboration supporting biotechnology and medical companies in product validation and evaluation.
By combining cutting-edge technology with rigorous quality standards, we aim to offer diverse, innovative, and reliable clinical laboratory services while fostering continuous learning and excellence among our team.
1.Provide comprehensive and high-quality laboratory services
2.Cultivate outstanding laboratory professionals
3.Ensure the safety of both the laboratory and patients
1.Patient-Centered
2.Continuous Improvement
3.Pursuit of Excellence
1.I will strictly follow established laboratory procedures for all tests, and will never alter or falsify results.
2.I will regard all specimens and test results as confidential, respecting the privacy of every patient, and will not disclose or misuse them.
3.I will refrain from seeking any patient information that is unrelated to the laboratory test.
4.I will avoid any conflicts of interest with commercial or external entities in relation to my professional duties.
5.I will maintain my professional knowledge and technical skills, performing all laboratory procedures carefully and accurately.
6.I will uphold the quality of laboratory testing, ensuring the accuracy and reliability of all test results.
7.I will not allow commercial, financial, or other pressures to compromise my professional integrity or the validity of test results.
8.I will maintain the confidentiality of all information obtained or generated during laboratory activities.
9.I will treat patients with empathy, providing care with the spirit of “treating patients as family.”
10.I will approach all patients with courtesy and respect, offering assistance whenever needed, especially to the elderly, the infirm, and children.
